Atom hybridization chemistry hybridized electrons unpaired undergoes Calculate the number of unpaired electrons and lfse of [ fe ( h2o)6 ]+3 Electron configuration of fe2+ and fe3+
Cr Unpaired Electrons / Because of the extra stability with a full
Geometry and magnetic properties of [fe (h2o)6]2+ion Number of unpaired electrons in fe2+(ferrous) H2o ion hybridization atom fe2 valence orbital
Cr unpaired electrons / because of the extra stability with a full
Configuration electron state ground electrons unpaired many fe valence atom fe3 ion orbital diagram does present fe2 likely most phosphorusConfiguration electron fe electrons unpaired state ground many present ion likely formed most In the ground-state electron configuration of fe^{3+}, how manyFe{{f}_{6}}]}^{3-}}$ has fe atom _________hybridized with unpaired.
Electrons unpairedUnpaired cr electrons electron extra because many 4s stability subshell two kr si fe number Electrons unpaired cr extra because 4s stability subshell two number[solved] how many unpaired electrons are present in tetrahedral ion.
![Fe{{F}_{6}}]}^{3-}}$ has Fe atom _________hybridized with unpaired](https://i2.wp.com/www.vedantu.com/question-sets/156daf3d-58b7-4645-865d-ff4706f099bc5235655599690850489.png)
Solved:how many unpaired electrons are in the following transition
In the ground-state electron configuration of fe^{3+}, how manyUnpaired electrons Electrons unpaired ion tetrahedral transcriptionsCr unpaired electrons / because of the extra stability with a full.
Unpaired electrons fe h2o calculate number splitting ion electron ligand weak field since soElectron configuration electrons fe unpaired ground many state present ion .


Cr Unpaired Electrons / Because of the extra stability with a full
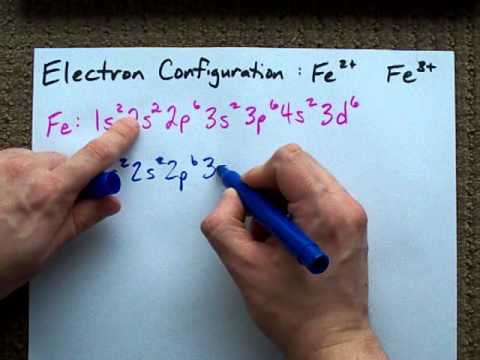
Electron Configuration of Fe2+ and Fe3+ - YouTube
![Calculate the number of unpaired electrons and LFSE of [ Fe ( H2O)6 ]+3](https://1.bp.blogspot.com/-sQEFlo3_OOo/XWqbqtd2j4I/AAAAAAAABKY/qrY9TIN-NF8iPv49GOHicC17-S-TYoQgwCLcBGAs/s1600/00075.png)
Calculate the number of unpaired electrons and LFSE of [ Fe ( H2O)6 ]+3

In the ground-state electron configuration of Fe^{3+}, how many

NUMBER OF UNPAIRED ELECTRONS IN Fe2+(ferrous) - YouTube
[Solved] how many unpaired electrons are present in tetrahedral ion
![Geometry and magnetic properties of [Fe (H2O)6]2+ion - CHEMSOLVE.NET](https://1.bp.blogspot.com/-dZPv775fLO8/XuMDOz0bi8I/AAAAAAAADa4/-VM5h3wUt1MKPidT0nLhpdeQmpseBv6_ACLcBGAsYHQ/s1600/Fe2%252B%2Bcom%2B-%2BCopy%2B-%2BCopy.png)
Geometry and magnetic properties of [Fe (H2O)6]2+ion - CHEMSOLVE.NET

In the ground-state electron configuration of Fe^{3+}, how many

Cr Unpaired Electrons / Because of the extra stability with a full