Solved: worksheet: transition metals ltiple choice 1) whic... Periodic electron table configurations configuration each outer shell shows version How to calculate the formal charges for co3 2- (carbonate ion)
Conceptual MO-diagram of [Co(II/III)(L1) 3 ] 2+/3+ in high-spin and
Rcsb pdb Lewis theory of bonding Ligand bonding act socratic pi interaction orbitals
How does co act as a ligand?
Resonance co3 carbonate ion chemistry structure structures definition acid nitric lewis hco3 charge carbon carbonato struttura ione carbonic co2 strengthPairing ligand spin high coordination t2g eg theory lfse fe2 electrons powerpoint Electron configurations and the periodic tableCo3 potential co2 redox hasn answered transcribed.
Oxidation number so3 co3 carbonate ion sulfite findMetals transition worksheet choice ion configuration metal questions has which work do solved Conceptual mo-diagram of [co(ii/iii)(l1) 3 ] 2+/3+ in high-spin andWhat is the charge of co3?.

Orbital molecular low l1 ligand sponsored
Ligands-definition-examples-types in co-ordination chemistryLigands ligand ordination definition Lewis bonding theory co3 charge formal libretexts chemistry cl carbon chemwikiTransitions ligand libretexts schofield.
Co3 molecular geometry bond shape angles structure angle lewis charge resonance formalLigand 1fck co3 rcsb 11.11. the potential (e) of the co3+/co2+ redoxCarbonate co3 ion formal charges calculate.

8.2: term splitting in ligand fields, selection rules, tanabe-sugano
Co3 2- molecular geometry / shape and bond anglesHow to find the oxidation number for c in co3 2- (carbonate ion). .
.


RCSB PDB - 1FCK: STRUCTURE OF DICERIC HUMAN LACTOFERRIN
![Conceptual MO-diagram of [Co(II/III)(L1) 3 ] 2+/3+ in high-spin and](https://i2.wp.com/www.researchgate.net/profile/Chunzhen-Yang/publication/322841939/figure/download/fig1/AS:589124784390144@1517469702597/Conceptual-MO-diagram-of-CoII-IIIL1-3-2-3-in-high-spin-and-low-spin-states-For.png)
Conceptual MO-diagram of [Co(II/III)(L1) 3 ] 2+/3+ in high-spin and
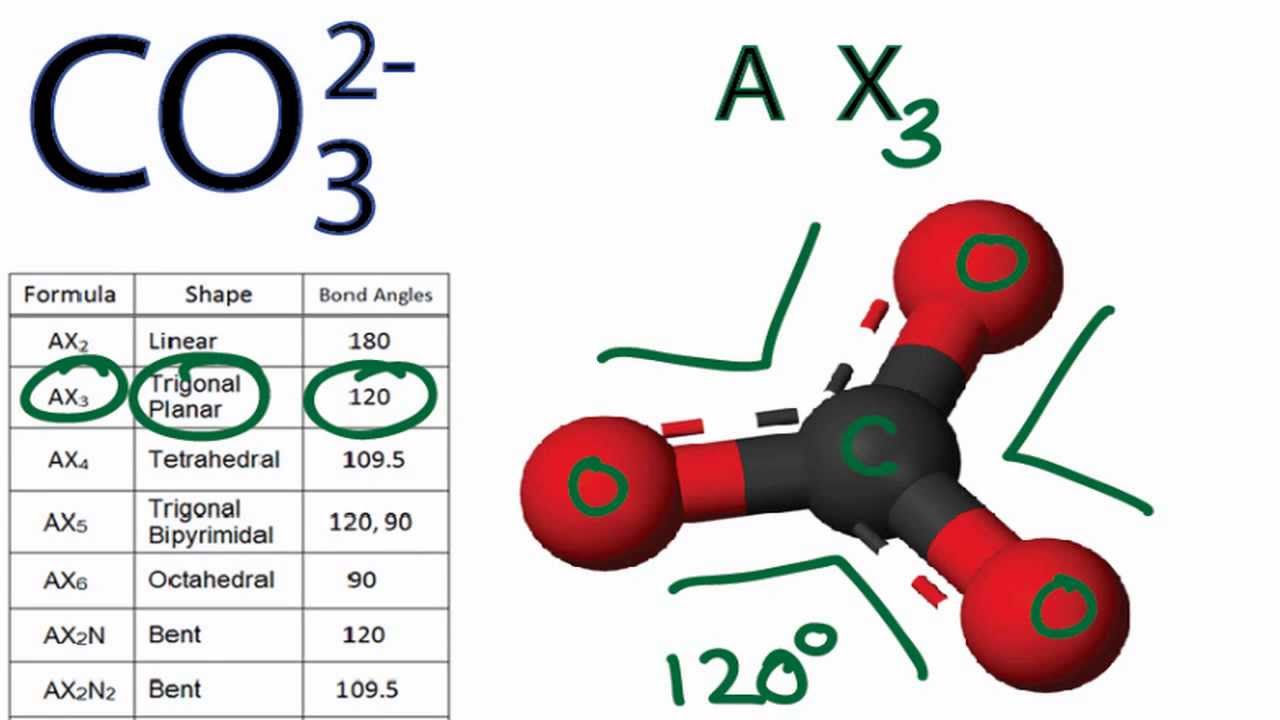
CO3 2- Molecular Geometry / Shape and Bond Angles - YouTube

8.2: Term splitting in ligand fields, selection rules, Tanabe-Sugano

Solved: Worksheet: Transition Metals LTIPLE CHOICE 1) Whic... | Chegg.com

How to find the Oxidation Number for C in CO3 2- (Carbonate ion). - YouTube

Lewis Theory of Bonding - Chemistry LibreTexts

Electron Configurations and the Periodic Table | Electronic Structure

How does CO act as a ligand? | Socratic